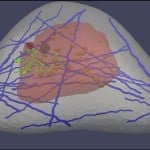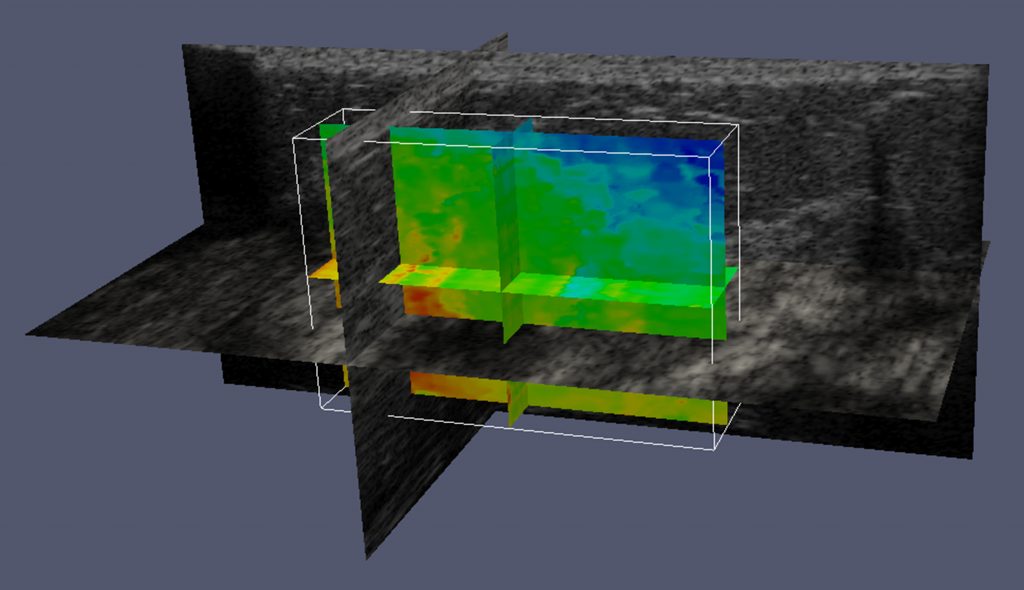
Jingfeng Jiang is the principal investigator on a project that has received a $450,187 research and development grant from the National Institutes of Health, “Elastography-Based Analytics for Benign and Malignant Breast Disease.”

Ultrasound elastography is used to pinpoint possible tumors and differentiate malignant, cancerous growths from benign lesions throughout the body, including in the breast. “Cancer tissues are stiff, and aggressively change their surroundings,” says Jiang, an associate professor of biomedical engineering at Michigan Tech.
“Ultrasound elastography uses imaging to measure the stiffness of tissue. Depending on who does the reading, the accuracy can vary from 95 percent to 40 percent,” Jiang says. “Forty percent is very bad—you get 50 percent when you toss a coin. In part, the problem is that ultrasound elastography is a relatively new modality.”
Ultrasound elastography could be an excellent screening tool for women who have suspicious mammograms, but only if the results are properly interpreted. Jiang’s research team, along with Zhengfu Xu, assistant professor of mathematical sciences, will use their graphics processing unit (GPU) to perform advanced processing of raw ultrasound data so physicians can use that information in their clinical workflow. “Mainly, radiologists will use our software together with ultrasound elastography and ultrasound for diagnosis,” says Jiang. “Our goal is to greatly reduce the guesswork.”