Chathura de Alwis (Chemistry), Timothy R. Leftwich (MSE), Pinaki Mukherjee (MSE), Alex Denofrea (Chemistry) and Kathryn A. Perrine (Chemistry) published a paper titled “Spontaneous selective deposition of iron oxide nanoparticles on graphite as model catalysts” in Nanoscale Advances in 2019.
DOI: 10.1039/c9na00472f
Extract
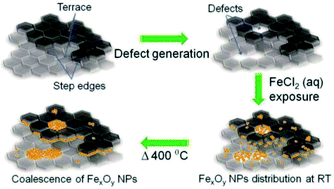
Iron oxide nanomaterials participate in redox processes that give them ideal properties for their use as earth-abundant catalysts. Fabricating nanocatalysts for such applications requires detailed knowledge of the deposition and growth. We report the spontaneous deposition of iron oxide nanoparticles on HOPG in defect areas and on step edges from a metal precursor solution.
Various defects were created on the highly oriented pyrolytic graphite (HOPG) surface using either argon (Ar+) sputtering or a focused ion beam (FIB) to provide defects for nucleation sites. A Hitachi 2000 A FIB instrument was used to create tailored arrays of defects on HOPG using a Ga+ beam.
The sputter rate was calculated using the amount of materials removed, by recording a height profile of 1 nm using atomic force microscopy (AFM) and the time to sputter the pattern.
All the samples were imaged using a Hitachi S-4700 cold field emission high resolution field emission scanning electron microscopy (FE-SEM) instrument.
X-ray photoelectron spectroscopy (XPS) was performed using a PHI 5800 to analyze the elemental composition and oxidation state of surface species of the iron oxide nanoparticles grown on the HOPG surface.
Scanning transmission electron microscopy (STEM) imaging and energy dispersive X-ray spectroscopy (EDS) mapping were used to measure the phase and composition of iron oxide nanoparticles after annealing and to confirm if the deposition was preferential at the defect sites of graphite. A FEI Titan Themis aberration corrected scanning transmission electron microscope was used to obtain atomically resolved electron images and EDS maps of the iron oxide nanoparticles on the graphene coated TEM grid. The microscope was operated at 200 kV using a point resolution of the aberration corrected STEM mode of 0.08 nm. The microscope was equipped with a SuperX™ X-ray detector, which is composed of 4 detectors for fast X-ray mapping in STEM mode. The EDS mapping of the sample was performed on specific particles with an average beam current of 100 pA.
Acknowledgements
Equipment for obtaining the AFM images in this project was provided by NSF CHE #1725818. The electron microscopy research was performed at the Applied Chemical and Morphological Analysis Laboratory, at Michigan Technological University. The electron microscopy facility is supported by NSF MRI 1429232. We acknowledge the Michigan Tech REF-RS fund for support of this work and the David J. and Valeria Pruett Graduate Research Fellowship. We acknowledge the Applied Chemical and Morphological Analysis Laboratory (ACMAL) for staff assistance and use of facilities.
Recommended Citation
de Alwis, C., Leftwich, T., Mukherjee, P., Denofre, A., & Perrine, K. (2019). Spontaneous selective deposition of iron oxide nanoparticles on graphite as model catalysts. Nanoscale Advances, 1(12), 4729-4744.
http://doi.org/10.1039/C9NA00472F
Retrieved from: https://digitalcommons.mtu.edu/michigantech-p/1246