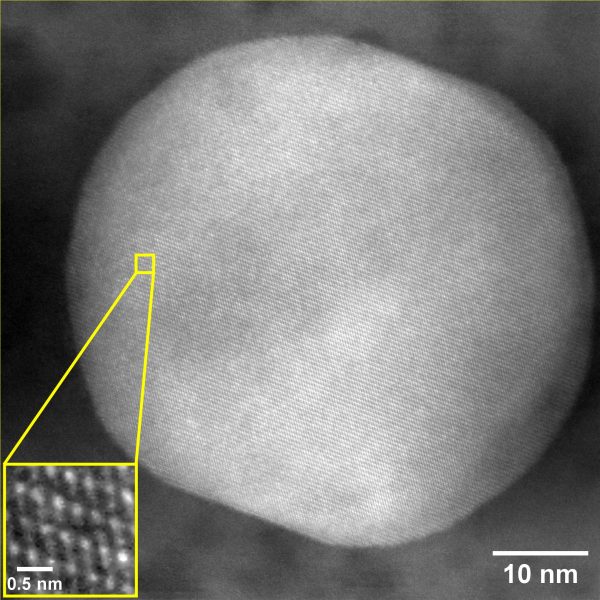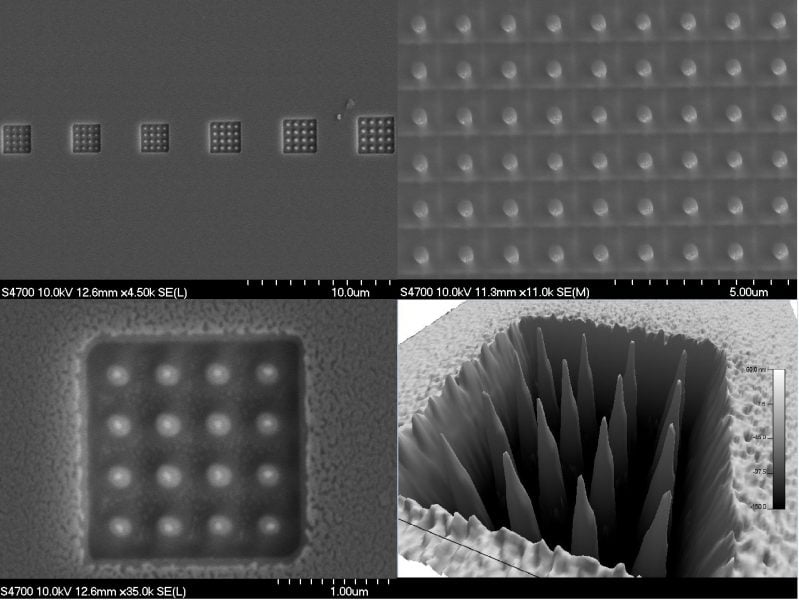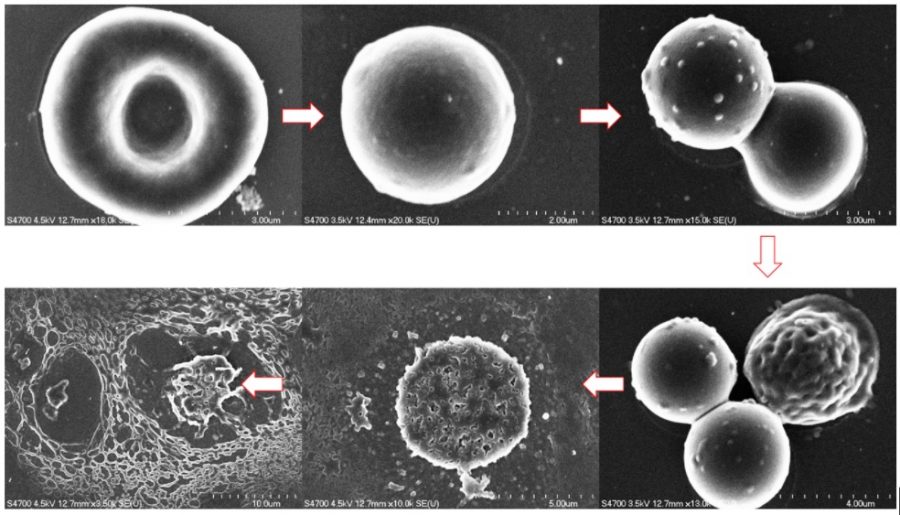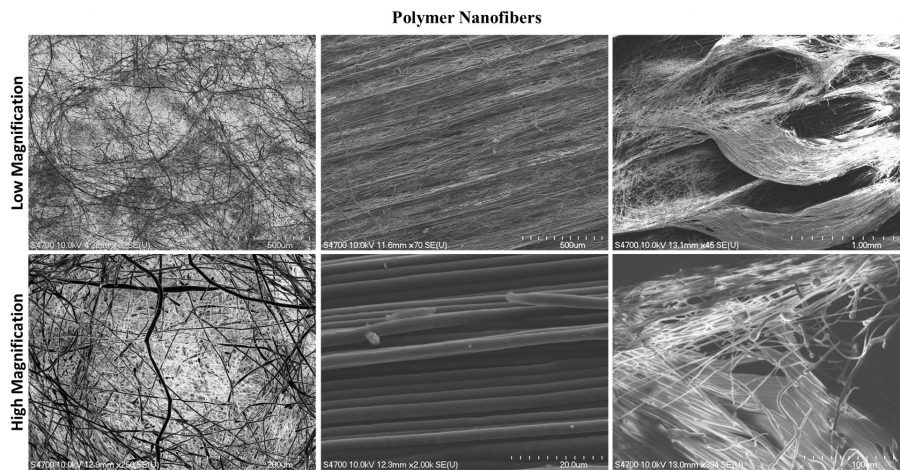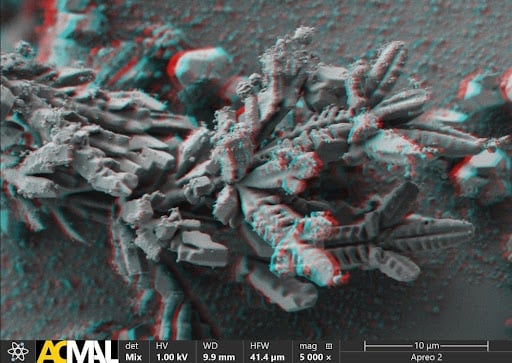
ACMAL staff and guests alike are making the decision to get trained on the Cryo-SEM. Getting trained on these instruments allows for the opportunity to use a scanning electron microscope to analyze frozen samples. A wide range of sample types can be used with this instrument including magnetic materials, powders, and biological materials. Using this method to examine biological material samples can be especially efficient because freezing them can leave them in a closer to natural state. In the most recent training, snowflakes were used as samples (as pictured).
Visit the Applied Chemical and Morphological Analysis Laboratory’s webpage to learn more about our shared facility and instruments available to the Michigan Tech research community.
To book a Cryo-SEM session or discuss a potential project contact: Elizabeth Miller – ACMAL Director Email: eafraki@mtu.edu.