
Tim Eisele and Neha Sharma generously shared their knowledge on Husky Bites, a free, interactive Zoom webinar hosted by Dean Janet Callahan. Here’s the link to watch a recording of his session on YouTube. Get the full scoop, including a listing of all the (60+) sessions at mtu.edu/huskybites.
What are you doing for supper this Monday night 3/15 at 6 ET? Grab a bite with Dean Janet Callahan and Tim Eisele, Associate Professor of Chemical Engineering at Michigan Tech. His focus: sustainable metallurgy.

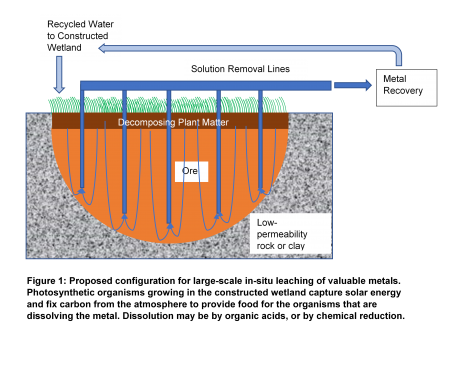
“There is more than one way to extract metals from ore,” says Eisele. “Massive mines that disrupt many square miles are not the only way to go. I have been working on a method for using bacteria to recover iron and manganese in such a way that, if it is done carefully, it may not even be obvious that mining is going on at all.”
Joining in will be Neha Sharma, one of Dr. Eisele’s PhD students. She came to Michigan Tech from the India Institute of Technology after internships at Tata Steel, the Julius Kruttschnitt Mineral Research Centre in Australia, and India’s National Metallurgical Lab.
Eisele holds a BS, MS and PhD in Metallurgical Engineering, all from Michigan Tech. In his research, he develops bacterial processes for upgrading and extracting iron ores and low-cost reprocessing of industrial wastes such as slags and sludges to recover valuable metals.
The inspiration for this began right in Eisele’s own yard, and in his own household well. “We have 9 acres of surprisingly varied property that includes rock outcroppings, grassland, woods, and a small fen–a type of wetland–that bleeds iron,” he explains.

“It all started when we bought the house. All the plumbing fixtures were stained red. Really red. I took a glass of untreated drinking water to my lab at Michigan Tech, and found that iron precipitated out. We struck iron! So I thought, ‘Why is this happening? Is there something constructive we can do with this?’”
The high iron content of his home well water, Eisele figured out, was caused by naturally occurring anaerobic iron-dissolving organisms.
“The UP is well known for having these elements in the soil, both iron and manganese,” says Eisele. Jacobsville sandstone is a visible example. The white lines in Jacobsville sandstone are where bacteria ate out the iron.”

Eisele cultivated anaerobic and aerobic organisms in the laboratory to fully adapt them to the ore. “We use mixed cultures of organisms that we have found to be more effective than pure cultures of a single species of organism,” he explains. “The use of microorganism communities will also be more practical to implement on an industrial scale, where protecting the process from contamination by outside organisms may be impossible.”
“There was not much initial interest in the technology from industry,” recalls Eisele. “‘If you can demonstrate that you can do it at a profit, come talk to us,” they said.
Since that time, Eisele and his team have been branching out to also extract manganese, which is dissolved by the same organisms as the ones that dissolve iron. This has attracted more interest, including a recent funded project from the U.S. Department of Energy.
“Manganese is one of the ‘battery metals,’” Eisele explains. “It’s also used heavily in most steel alloys.”
“Manganese is also currently considered a ‘critical element”. Currently there is no manganese mining or production in the US,” adds Eisele. “While there are manganese ores in this country, new extraction technology is needed in order to be competitive with ores elsewhere in the world.”
In Eisele’s lab at Michigan Tech, Neha Sharma and other students, both graduate and undergraduate, work on developing and refining the technology. This includes a small “model wetland” consisting of a 5-gallon container with a circulation of water and appropriate nutrients, –in effect, simulating the type of wetland that leaches metal.
“I work on a manganese leaching setup,” Sharma explains. “It involves analyzing the samples we get from the leaching flasks for the presence of manganese. The best part of the work? “New findings are always the best part,” says Sharma. The most challenging? “Writing about them!”

Professor Eisele, how did you first get involved in engineering. What sparked your interest?
I have been interested in science and engineering for as long as I can remember. I originally decided to work with metals after taking a welding class in high school, and came to Michigan Tech to study metallurgy in 1980.

Family and hobbies?
I grew up on a small dairy farm in the Thumb area of lower Michigan, near Kinde (population 400). I then decided to move here, to the Big City. I currently live just outside of town with my wife, two children, a dog, a cat, six chickens, and a variable number of beehives. My daughters are still in school, and my wife is a locksmith.
“In my spare time, I like to take photos of insects, and started a website about it back in 2007, The Backyard Arthropod Project. Both of my daughters have participated in this from the beginning, and neither of them has the slightest fear of insects or spiders. My older daughter’s first contribution at the age of 2 was an assassin bug nymph, that she brought while crowing, ‘Take picture, Dada!’ My younger daughter, also at the age of 2, brought me a nice longhorn beetle that she held up while calling out ‘See! Bug!’ Lately I’ve also been including postings about the local plants, and have a couple of posts about the metal-leaching properties of our wetland.”

Neha, how did you first get involved in engineering? What sparked your interest?
“I was always interested in science during my school days, so when I graduated from high school I thought that engineering would be the perfect fit for me. My major during my undergraduate studies in India was mineral processing. Working through those subjects and various internships –all focused on mineral processing and metallurgy–sparked my interest towards the sustainable aspect of these industries.”

Family and hobbies?

“I grew up in a small town in India called Bokaro Steel City. I earned my bachelor’s degree from the Indian School of Mines (now Indian Institute of Technology) in Dhanbad, India. My parents still live in India. My father is a teacher in high school, teaching math and physics. My older brother works for Borealis AI, in Canada. My mother is a homemaker and loves gardening. I love going to new places. In my spare time, I’ll read a book or sketch. I love badminton, and cross country skiing, too. I am also a big Lord of the Rings fan, and a Potterhead too!”